|
Investigation of the biofiltration potential of the Pacific Oyster (Crassostrea gigas) to decrease fecal coliform bacterial levels By Hannah Gray
Introduction
The Morro Bay estuary, located on the central coast of California, encompasses about 2,300 acres of tidal wetlands, mudflats, eelgrass beds, and open water habitat (MBNEP, 2001). Being one of the last natural estuaries between and Monterey Bay and San Diego, Morro Bay's rich mud flats and eel grass beds are vital habitats needed to maintain numerous aspects of the Pacific Coast ecological system and subsequently the local economy through support of the large commercial fishing, aquaculture, and tourism industries (MBNEP, 2001). The Morro Bay National Estuary Program (MBNEP) is a local organization whose mission is to protect the resources and water quality of the bay, while preserving its economic and recreational viability. One of Morro Bay's high priority problems, as identified by the MBNEP based on year-round monitoring, is high bacterial concentrations (MBNEP, 2001). High coliform counts have been documented during both summer and winter months since as early as 1974 (MBNEP, 2000). Bacterial contamination is impacting shellfish aquaculture operations within the bay to the extent that harvesting is currently restricted in about one quarter of the lease area, and other areas are closed to harvesting during periods of high runoff (MBNEP, 2001). In addition to economic impacts, high coliform concentrations in recreational areas are known to pose a health risk to those in contact with the water (MBNEP, 2000). Possible sources of bacterial contamination include agricultural runoff, urban runoff, leaking or failing septic systems, illegally moored boats with inadequate waste disposal capabilities, `mestic animal waste and waste from marine mammals and wildlife (MBNEP, 2000). The impact of some of these sources can be reduced, though not eliminated, through various management plans. Additionally, implementation and regulation of these plans is often a costly process that can take many years to put into place; in nearby Los Osos the installation of a sewer system and waste water treatment plant, designed to eliminate bacterial contamination from the septic tank systems, is a project that has been pending for 20 years. There is a clear need for a more immediate method of reducing bacterial concentrations until proposed management plans can be fully implemented. The use of a biological filter could be an effective tool to fill this need. By looking at the definitions of the words "biological" (caused by a living thing) and "filter" (removes something from whatever passes through it), a biological filter can be defined as a living thing that removes something from whatever passes through or by it (Dictionary of Bioscience, 1997). The viability of using a known filter-feeding organism, such as a bivalve, to perform the task of filtering out bacteria from the water column, would be worthy of further exploration. Bivalves have been proven to act as effective biofilters in a number of situations. In 1982, South San Francisco Bay was receiving about 70 percent of the sewage effluent from the city, and the bottom-dwelling filter feeders including bivalves were thought to be filtering out enough of the nutrient particles to suppress accelerated eutrophication (Officer et al, 1982). With the invasion of zebra mussels (Dreissena polymorpha) into the Hudson Bay 1991, a marked decrease in the concentrations of cyanobacteria, as well as other plankton, was observed. By 1993, the increasing number of zebra mussels in the bay peaked and leveled out; their estimated filtration rates at that time corresponded to theoretical turnover times of 1.2 to 3.6 days for the entire estuary (Strayer et al, 1999). In Morro Bay, high coliform concentrations have been observed outside the oyster bed areas as well as in the oyster's flesh, but often not in the water over the oyster beds (MBNEP, 2001). The Pacific oyster (Crassostrea gigas), has been shown to be reasonably efficient in particle selection (Kiørboe and Møhlenberg, 1981) and is more resistant to disease than other bivalves (Gottlieb and Schweighofer, 1996). C. gigas has also been proven capable of filtering out and retaining pathogens such as Salmonella (Plusquellec et al, 1994). Additionally, the Pacific oyster has a long and well-known track record in Morro Bay, showing it survives well and has a fully controllable population in environmental conditions found here. Given this information, C. gigas is a practical choice as biofilter for bacterial contamination in Morro Bay. The MBNEP and the Environmental Protection Agency use total fecal coliform and Escherichia coli concentration numbers as an indicator of the presence and concentration of disease-causing bacteria. Little research has been done on the ability and efficiency of Crassostrea gigas to filter specific particles, such as fecal coliforms, out of the water column. This basic information is vital for any application that utilizes the biofiltration abilities of C. gigas. The project proposed here is designed to determine how well C. gigas can filter E. coli and fecal coliforms when placed in an environment with conditions similar to those found in Morro Bay. The results of this research will provide substantial evidence to support the viability of a larger scale field study to determine if C. gigas can indeed function as an effective, short term biofilter for bacterial contamination until long-term management plans can be fully implemented.
Materials and Methods Oysters were placed in a circular, recirculating tank of sterile seawater. A known amount of Escherichia coli was added to the tank with oysters and an identical control tank with no oysters. Water samples were taken from both tanks at set intervals over a span of 15 hours. E. coli levels in each sample were recorded and plotted to show the rate of decline in bacterial concentrations. The rate of decline in the oyster-containing tank was compared to that of the control tank to determine how much bacteria the oysters are filtering out of the water column. The experiment was repeated three times to ensure accuracy. Experiments were carried out between December 2000 and January 2001. Crassostrea gigas specimens were purchased from B.J. Enterprises (local live seafood supplier). When not being used in experiments, oysters were kept submerged a netting box by the Morro Bay Coast Guard pier where there was adequate water flow to keep the oysters well fed and healthy. Filtered, sterilized fresh seawater collected from the Tidelands Park launch ramp was used in all experiments. The sterilization process consisted of pumping water through a 5-micron filter and a UV sterilizer. A student-safe strain of Escherichia coli was purchased from Carolina Biological Supply Co.; each tank was inoculated with 5 ml of a 24-hour pure broth culture (approximately 150 x 106 cells). Sterile lab procedures were used when handling all infected water and tank equipment. Control and experiment tanks were torus-shaped, each made out of a sliced truck tire with an upside-down flowerpot stuck up through the middle, all coated with fiberglass. Both tanks had an overall diameter of 1 meter, a trough width of 0.35 meters and a depth of 0.35 meters. Rio 180 water pumps were used in both tanks to maintain a constant water flow. A system involving a tube with random small holes attached to a Rio 800 pump was used to keep particulate matter from accumulating on the bottom of each the tank. The Colilert® 18 hr MPN method was used to quantify E. coli concentrations. Samples were taken at intervals over a 15 hr period. A sample dilution of 1:1000 was used to achieve measurable results. Data was kept in Microsoft Access, graphs and statistical work were done using Intuitive Logic's ClickIt .
Results The results of Escherichia coli concentration monitoring in both tanks are presented in tables 1, 2, 3 & 4 (fig. 1). In each trial, the oyster tank bacteria levels dropped 50% ± 10 in the first three hours, compared to 30% ± 5 drop in the control tanks. Data shows, in all three trials, the oyster tank E. coli levels continue to drop more rapidly than the control tank levels. A graph of the geometric mean of bacterial concentration declines is shown in figure 2. The graph plots the bacterial concentrations as a percent of original over in each tank over a period of 15 hours. The curve of best fit for bacterial concentrations in each tank is shown on the graph in grey, and can be represented as an inverse exponential function of time (fig. 3). 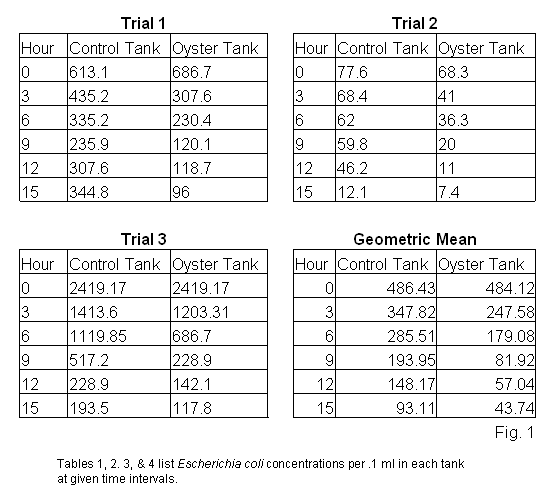 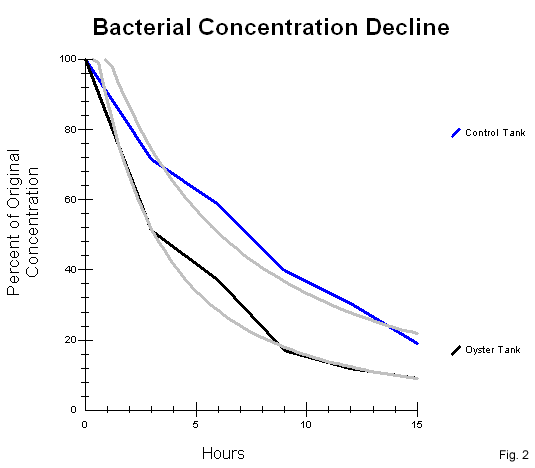 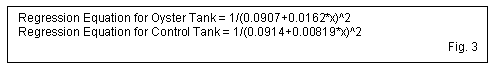
Discussion
During the course of the experiment, some problems were encountered. The tanks were kept outside to keep the water cold, as no chiller was available. It was discovered, however, that at average evening temperatures of 5.5-7º C, only about 5-10 of 50 oysters opened their valves. When water was heated to 11.1º C about 40-45 of the oysters were open at any given time. Open to closed ratios above 45:50 were not observed at any temperature. Prolonged (6-12 hours) overheating of the tank to and over 21.5º C resulted in a 90% fatality rate. Some trial-and-error testing was necessary to rig and calibrate a functioning heating system that maintained a constant temperature of 12 ± 1.8º C. Differences in initial Escherichia coli levels were due to inaccurate methods (comparing culture to a #1 tube on the nephelometer scale) of quantifying the density of the 24-hour broth culture. The use of a spectrometer would help to measure and subsequently standardize the amount of bacteria added to each tank. Another concern that came up was the difference in flow rates between the two tanks due to the drag created by the oysters. In future experiments, large rocks will be placed in the control tank to simulate the flow interruptions caused by the oysters. Results of the experiments show a notable difference between the bacterial concentration decline rates of the control and oyster tanks. The decreased bacterial concentrations in the oyster tank is likely due to the filtering of Escherichia coli by Crassostrea gigas. This conclusion is supported by the work done by Plusquellec that showed C. gigas's ability to filter Salmonella and Strayer's observations of changes in the Hudson River ecosystem due to the introduction of the zebra mussel. Evidence from the study presented here substantiates further exploration into the possible use of C. gigas as a biofilter for fecal coliform contaminates. A recently obtained grant from the Morro Bay National Estuary Program will make continued research possible. Other variables, such as temperature, turbidity, and substrate will be adjusted to find out if and/or what affects they have on the filtration rates of Crassostrea gigas. Trials will be done over a period of 32 hours instead of 15 to trace the decline rate further down. The results of this in-depth study should shed more light on the filtering abilities of C. gigas, which in-turn will provide key data needed to decide if C. gigas can function as an effective biofilter for fecal coliform contaminates in Morro Bay.
References
Morro Bay National Estuary Program, 2001. <http://www.mbnep.org/about.htm> Morro Bay National Estuary Program, 2000. Coastal Commission Management Plan. Parker, Sybil P. Ed.,1997. Dictionary of Bioscience. McGraw-Hill. New York, NY, pp. 61 & 176. Officer, C. B., T. J. Smayda, R. Mann, 1982. Benthic filter feeding: A natural eutrophication control, Marine Ecology Progress Series. 106, pp. 147-156. Strayer, D. L., M. F. Caraco, J. J. Cole, S. Findlay, M. L. Pace, 1999. Transformation of freshwater ecosystems by bivalves: A case study of Zebra Mussels in the Hudson River, BioScience. 49(1), pp. 19-27. Kiørboe, T., F. Møhlenburg, 1981. Particle selection in suspension-feeding bivalves, Marine Ecology Progress Series. 5, pp. 291-296. Gottlieb, S. J., M. E. Schweighofer, 1996. Oysters and the Chesapeake Bay Ecosystem: A case for exotic species introduction to improve environmental quality?, Estuaries. 19(3), pp. 639-650. Plusquellec, A., M. Beucher, C. Le Lay, D. Gueguen, Y. LeGal, 1994. Uptake ad retention of Salmonella in bivalve shellfish, Journal of Shellfish Research. 13(1), pp. 221-227. |